The multi-disciplinary approach for the diagnosis of multiple myeloma
Multiple myeloma is the malignant growth of plasma cells that accumulate in the bone marrow, leading to bone destruction and marrow failure.
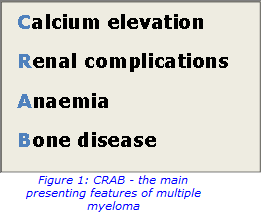
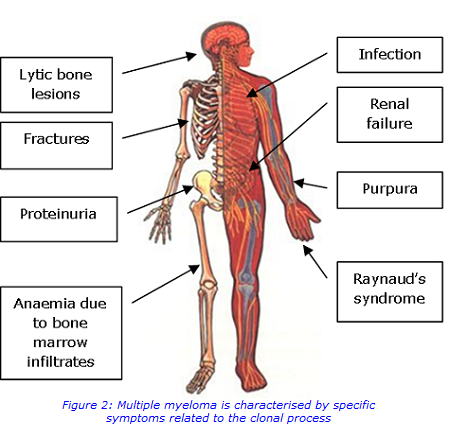
Plasma cells play a central role in the immune system. They develop from B-cells (a type of white blood cell) and produce large amounts of immunoglobulins (antibodies). Multiple myeloma occurs when abnormal plasma cells escape control and expand rapidly beyond the constraints of normal cell death. The most common symptoms include bone pain, recurring infection, kidney damage and fatigue. The acronym CRAB, shown in Figure 1, is used to identify the main presenting features.
What causes myeloma?
The causes of myeloma are not fully understood but it is thought to develop due to an interaction between both genetic and environmental factors.
Myeloma usually evolves from an asymptomatic pre-malignant stage of clonal plasma cell proliferation termed monoclonal gammopathy of undetermined significance (MGUS). MGUS is present in more than 3% of the population above the age of 50 and progresses to myeloma or related malignancy at a rate of 1% per year.
Laboratory tests
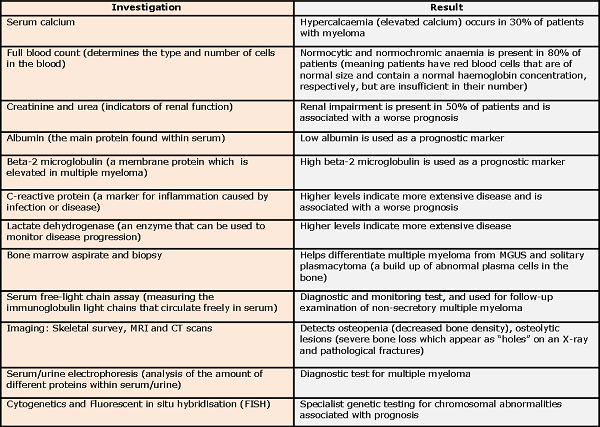
A multidisciplinary approach is important for the diagnosis of multiple myeloma, with laboratory tests from Biochemistry, Haematology, Immunology and Genetics all providing key results.
Diagnostic tests for multiple myeloma Electrophoresis:
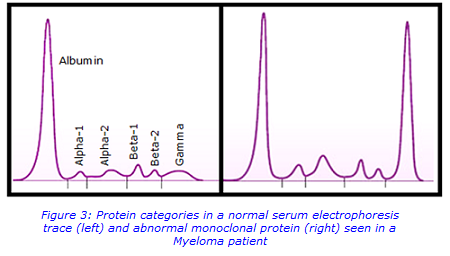
Protein electrophoresis separates the proteins in a blood sample into several groups based on their electrical charge and size. Albumin is the major protein component of serum and appears as the biggest peak that lies closest to the positive electrode. Globulins comprise of a much smaller fraction of the total serum protein but are the primary focus of interpretation. The gamma fraction lies closest to the negative electrode.
Figure 3 shows the protein categories in a normal trace compared to the trace of a myeloma patient. In the latter, a dense, narrow band composed of a single type of immunoglobulin can be seen in the gamma region. This is secreted by an abnormally expanded clone of plasma cells and is known as a monoclonal protein (M-protein, paraprotein). Highlighting the area of abnormality, and looking at the percentage of total protein this area represents, allows quantification of the protein to be given in g/L.
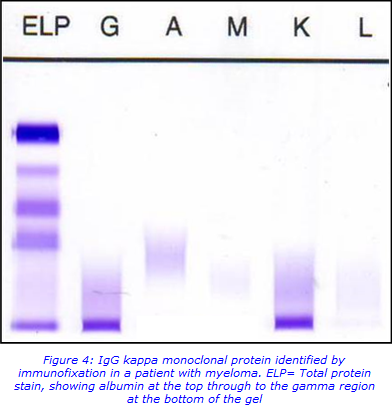
Upon detecting an abnormal monoclonal protein, immunofixation can be used to identify the heavy and light chain of the clonal immunoglobulin (Ig). In this method, serum samples are electrophoresed in agarose gel and then specific antibodies are added to the gel to bind to corresponding proteins before staining the gel. Visual identification of the heavy chain (IgG, IgA, IgM, IgD, and IgE) and the corresponding light chain (kappa, lambda) is then possible. Figure 4 shows an example of an immunofixation gel, with the applied antisera, containing the specific antibodies, labelled above each.
Fluorescent in situ hybridisation (FISH):
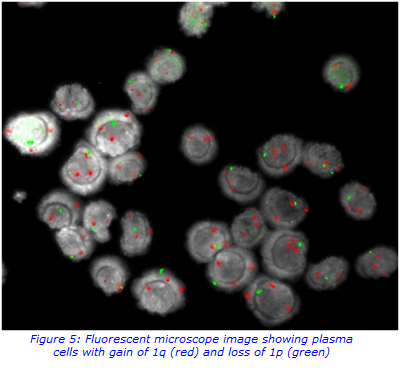
FISH uses fluorescent probes that bind to specific regions of the cell’s DNA to identify gains, losses and rearrangements of the genetic material.
The plasma cells in bone marrow samples are positively selected for the CD138+ markers on the outside of the cells. These plasma cells are collected and processed to allow hybridisation of the FISH probes, as shown in Figure 5.
The results provide an indication of the prognosis. Clinicians use this information to help with the diagnosis and also aid in treatment regimens for the patient.
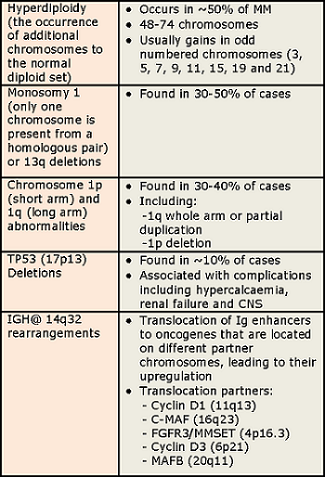
Abnormalities include:
Treatments available
Improvements made to treatment over the last decade have meant that survival rates in myeloma are increasing at the fastest rate among all cancer types in the UK.
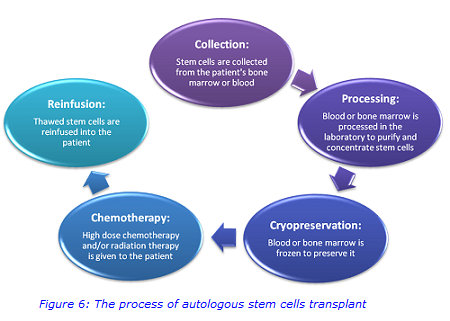
In the past decade, increased use of proteasome inhibitors (drugs that prevent the activity of proteasome complexes that break down proteins) and immunomodulatory drugs (which are able to suppress the immune system) have improved the outlook for myeloma patients; however the disease still remains incurable. Triple drug combinations are routinely used in induction regimes, followed by autologous stem cells transplantation in appropriate patients as shown in Figure 6.
Other treatments will also be prescribed to help prevent or manage potential side-effects and treat the symptoms and complications of myeloma. The final goal should be to find a balance among efficacy, toxicity and cost as well as the dream of achieving the cure for this disease.
For further information regarding the genetic tests, please contact:
www.viapath.co.uk/departments-and-laboratories/genetics
Genetics Department
5th Floor Tower Wing
Guy’s Hospital
Great Maze Pond
London
SE1 9RT
Sample requirements for genetic tests:
Cytogenetic analysis in plasma cell myeloma is carried out by interphase fluorescence in situ hybridisation (FISH) analysis of CD138 positive cells enriched from bone marrow aspirate samples.
Sample type: bone marrow aspirate in bone marrow transport medium. Do not spin down or freeze samples before sending. Samples must arrive within 24 hours.
Current service turnaround times: 5-10 calendar days (National targets: Non-urgent - 95% reported within 21 days)
For more information about the immunology tests, please contact:
kch-tr [dot] immunology [at] nhs [dot] net
Diagnostic Immunology and Allergy Department
King's College Hospital
Bessemer Wing - 1st Floor
Denmark Hill
London
SE5 9RS
Sample requirements for immunology tests:
Serum/urine protein electrophoresis, Immunofixation/Immunotyping serum free kappa and lambda light chains.
Sample type: clotted (gold top vacutainer)
Volume: 4mL
Turn around time: 3 days (Immunofixation 1 week)

