Carbohydrate Deficient Transferrin: A Marker of Chronic Excessive Alcohol Consumption
What is Carbohydrate Deficient Transferrin (CDT)
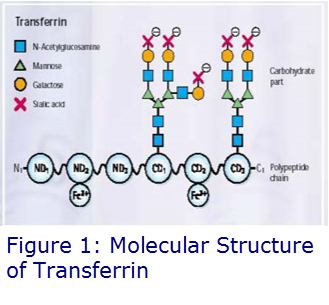
Transferrin is a 80kDa glycoprotein, synthesised in the liver and is the main iron transporting protein in the blood. The molecule is a single polypeptide with two N linked-side-chains. After its amino acid sequence has been formed, transferrin undergoes further modification by the addition or removal of carbohydrate (sugar) side-chains. This process is under the control of two enzyme systems; one which adds the side-chains (glycosyl transferases) and one which removes them (sialidases). This results in transferrin being exhibited in various isoforms and, based on the sialic acid content, there are up to seven of these, 3-5 of which can appear in the blood of normal subjects.
Carbohydrate Deficient Transferrin (CDT) is the term used for the group of isoforms of transferrin which are deficient in sialic acid residues, hence the term ‘carbohydrate deficient transferrin’. The asialo (without a side-chain), monosialo (1 side-chain) and di-sialo (2 side-chains) isoforms of transferrin are collectively called CDT.
Alcohol appears to have a direct effect on the enzyme systems involved in regulating the transferrin side-chains; it inhibits the enzymes adding the side-chains and stimulates the enzymes removing the side-chains. Thus excessive and repeated alcohol consumption modifies the isoforms’ distribution, resulting in an increase of the less sialylated forms, disialo and asialo (CDT). This means that CDT can be used as a marker of excessive alcohol consumption
CDT—A Marker of Chronic Excessive Alcohol Consumption
The most accurate way to use CDT as a biomarker for excessive alcohol consumption is to express its concentration as a percentage of total transferrin, thereby avoiding falsely high or low test results that may be due to very high or low total transferrin concentrations, and this utilisation of %CDT is approved by the US Food and Drug Administration (FDA) as a clinical diagnostic test for the detection of heavy alcohol consumption.
In normal individuals, CDT comprises of less than 1.6% of the total transferrin concentration. However, individuals that misuse alcohol typically have a higher proportion of transferrin as CDT (>2%) but, as the half-life of the protein is 7-28 days (i.e. the time it takes for the protein to be cleared from the circulation), the excess intake of alcohol would need to fall within this time period.
CDT is not a good marker of occasional excessive alcohol intake or binge drinking. However, CDT is a better marker of excessive alcohol misuse than the traditionally used markers of γ-glutamyl transferase (GGT) and mean corpuscular volume (MCV), due to the fact that it is not affected by mild alcohol-related liver disease, fatty liver (due to obesity, diabetes etc), chronic disease or B12/folate deficiencies.
It had been reported that in subjects potentially misusing alcohol CDT has 95% specificity i.e. 19 out of 20 times the increase in CDT level is due to excess alcohol intake.
There are three major genetic variants of transferrin in humans
1. Type C: common form with a prevalence 95- 97 %
2. Type B: extremely rare (only 20 cases so far identified) but causes false low CDT values
3. Type D: rare (less common in Caucasians but more common in Africans) but causes false high CDT values.
Types B, C, D can be separated on the basis of their charge but are indistinguishable antigenetically. Therefore, it is recommended that analytical methods based on charge separation are used in CDT testing, rather than immunoassays, to reduce the interference from type C and D variants.
Method Used to Measure CDT at Viapath
The Capillary Electrophoresis (CE) method (Capillarys 2, Sebai) is used to for CDT testing at Viapath. The CE provides a visual account of all isoforms present in the sample enabling determination of potential interferences such as paraproteins and complement degradation products. This method also enables the identification of the genetic variants of transferrin that may give false positive CDT value.
CDT Service at Viapath
A CDT service has been offered by the Metabolic Section of Viapath’s Reference Biochemistry Laboratory for more than 10 years and this service is available to both internal and external users. Presently Viapath is providing this service to the Driving Vehicle Licencing Agency (DVLA) and is used as an important part of the decision-making process for the reissuing of driving licences.
CDT testing is run every day and TAT is two working days. Last year 26,000 tests were performed.
Sample Requirements and Stability
Only serum should be used as EDTA/Heparin anticoagulants may disturb Fe3+ in-vitro TF saturation and may give false positives. Also, a blood sample stored unseparated for more than four days may give a false positive result so the sample should be separated within 2 hours of collection and then the serum sample is stable for 1 week if stored at 4C or for a longer period if stored at -20C
For any Clinical enquires please contact
Dr Hagosa Abraha: h [dot] abraha [at] nhs [dot] net
For sample requirement or any further enquires contact the Metabolic Laboratory or CDT Test