Assessment of a Promising New Drug for Sickle Cell Disease
What is Sickle Cell Disease?
Sickle Cell Disease is an inherited disease of haemoglobin and gets its name from the resulting, unusually shaped, red blood cells. The disease is a serious, lifelong condition, associated with anaemia, intermittent episodes of pain and other acute and chronic complications including stroke and renal failure, but there are very few treatment options available.
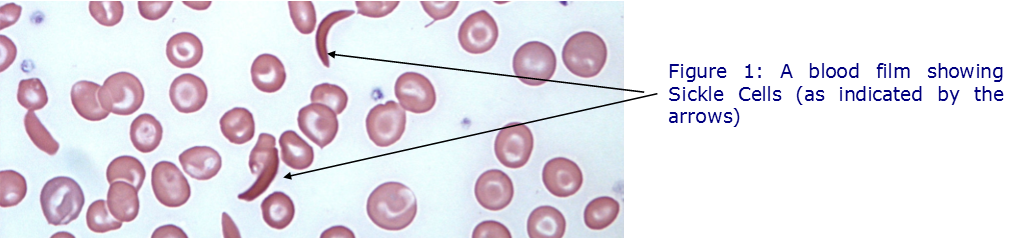
A New Drug Trialled
Viapath’s Special Haematology Team, at Guy’s Hospital, have been involved in a randomized, placebo-controlled, double-blind, phase I/II clinical trial with a new treatment drug called GBT440. The results were promising as the drug was well tolerated by the patients who exhibited decreased haemolysis and increased haemoglobin levels with an increased affinity for oxygen shifting the curve towards normal. GBT440 will start a phase 3 clinical trial in 2017.
The results from the initial drug trial were presented at the American Society of Hematology meeting in December see: https://ash.confex.com/ash/2016/webprogram/Paper95146.html.
How was the Efficacy of the Drug Measured?
One of the effects of GBT440 is to increase the oxygen affinity of haemoglobin and so the initial trial used P50, a measurement of the affinity of haemoglobin for oxygen, as an indicator of the efficacy of the drug . This functional assay produces an oxyhaemoglobin dissociation curve, which relates oxygen saturation and partial pressure of oxygen in the blood (PO2), and hence is used to indicate whether the drug has altered the haemoglobin’s oxygen affinity. The PO2 at which the haemoglobin is 50% saturated, is known as the P50, the higher/lower the value, the greater the affinity of the haemoglobin for oxygen, and allows an assessment of how readily oxygen is taken up and released to the tissues by the red cells.
P50 Analysis in Patients with Unexplained Erythrocytosis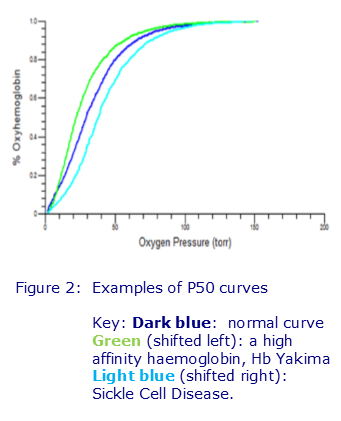
The P50 analysis for the GBT440 trial was carried out in the Viapath Special Haematology Laboratory at Guy’s Hospital, one of the few centres offering this test in the UK. Although the P50 measurement was an important factor in assessing the efficacy of the GBT440 in the phase I/II trial, in clinical practice it is more frequently requested to exclude a high affinity haemoglobin as part of the recommended investigations for patients with unexplained erythrocytosis and guidelines can be accessed at:
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2141.2005.05535.x/full
Please contact the laboratory for any enquiries regarding P50 analysis on: 0207 1883 421
Or via email:
Dr Yvonne Daniel, Consultant Scientist: Yvonne [dot] daniel [at] viapath [dot] co [dot] uk
Tracey Mare, Senior Biomedical Scientist: Tracey [dot] mare [at] viapath [dot] co [dot] uk
Daniel Dos Santos Monteriro, Senior Biomedical Scientist: Daniel [dot] monterio [at] viapath [dot] co [dot] uk
Leanne Kelly, Senior Biomedical Scientist: Leanne [dot] kelly [at] viapath [dot] co [dot] uk

