Skewed T Follicular Helper Cell Subsets in Common Variable Immunodeficiency
What is common variable immunodeficiency?
Common variable immunodeficiency (CVID) is one of the most frequent, clinically significant primary immunodeficiencies. It is a heterogeneous collection of disorders characterised by impaired antibody secretion (1-4). As a consequence of the failure to produce protective levels of antibodies, sinopulmonary infections are very common, with approximately 95% of patients suffering with pneumonia, bronchitis or sinusitis (2), and so chronic lung disease and bronchiectasis are frequently encountered complications. Patients with CVID therefore require replacement antibody therapy in order to improve their quality of life, reduce the frequency of infections and enhance their survival (5). However, there are also subsets of CVID patients that present with a range of other clinical features including malignant, inflammatory and autoimmune conditions (6). These associated conditions are not improved by antibody replacement, can be difficult to treat due to the need for immunosuppressive therapies and are now thought to be the main cause of morbidity and mortality (3). Hence there is a need to accurately stratify CVID patients.
The role of T follicular helper cells
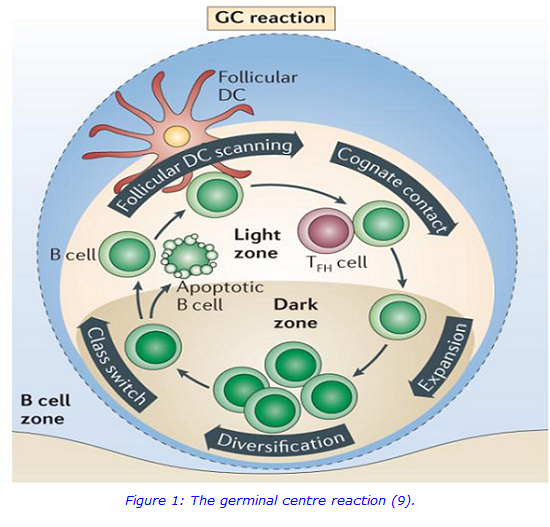
In an effective antibody response (Figure 1), antigen specific B cells are stimulated to proliferate in germinal centres, where they undergo affinity maturation and class switching. However, this germinal centre reaction may be defective in some CVID patients who are often found to have decreased levels of class switched memory B cells (smB) (5-8).
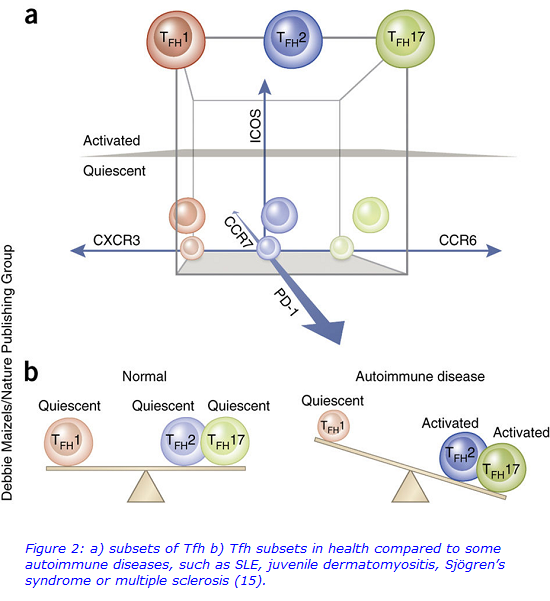
The importance of T follicular helper cells (Tfh) in the germinal centre is well known (10), where they provide numerous signals to the germinal centre B cells (11). Whilst Tfh are found in the germinal centres by definition, it is recognised that Tfh have a counterpart present in the circulation, meaning whole blood samples can be used to more easily study Tfh (12). Tfh are differentiated from the other T helper cell lineages as they uniquely express chemokine C-X-C motif receptor 5 (CXCR5), which enables migration into the B cell follicle, and they are further subdivided based on the presence of co-expressed CXCR3 and C-C motif chemokine receptor 6 (CCR6) to define three Tfh subsets: Tfh1, Tfh2, and Tfh17 (12,13). Given that the Tfh subsets differentially influence class switching and antibody secretion, the aim of the present study was to determine whether Tfh subset defects were present in CVID (12).
T follicular helper cells and CVID
As the Tfh and their subsets are easily distinguishable by a set of cell surface receptors, flow cytometry is an ideal method to use for their analysis. Circulating Tfh subsets were compared between 31 healthy controls and 24 CVID patients, using a novel whole blood, 9 colour flow cytometric assay and a Beckman Coulter Gallios flow cytometer. The assay also incorporated PD1 and ICOS expression to interrogate the activation status of each Tfh subset (15). Samples were also sent to University Hospital of Wales, Cardiff, for characterisation of B cells.
It was hypothesised that skewed Tfh subsets may provide a mechanism to explain the heterogeneity found in CVID patients, given that different Tfh subsets, like T helper cells, are stimulated by different signals received from the microenvironment and secrete different cytokines (11). Furthermore, dysregulation between the subsets has already been noted in several autoimmune conditions (Figure 2), but there is a paucity of data applying Tfh subset analysis to primary immunodeficiency (12).
For further information, please contact:
Charlotte Lee, Pre-registration Clinical Scientist, Cytogenetics
Email: charlotte [dot] lee10 [at] nhs [dot] net
At the IBMS Congress, 2017, Charlotte Lee submitted this work as poster and won the Immunology Category Award. The Immunology research group will be publishing their work in the near future and revealing their findings.
References:
1. de Vries E. Patient-centred screening for primary immunodeficiency: a multi-stage diagnostic protocol designed for non- immunologists. Clinical & Experimental Immunology. 2006;145(2):204–14.
2. Bonilla FA, Geha RS. Common variable immunodeficiency. Pediatric Research. 2009;65(5):13–9.
3. Cunningham-Rundles C. The many faces of common variable immunodeficiency. Hematology. 2012;1:301–5.
4. Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Journal of Allergy and Clinical Immunology. 2015;136(5):1186–205.
5. Ameratunga R, Brewerton M, Slade C, et al. Comparison of diagnostic criteria for Common Variable Immunodeficiency Disorder. Frontiers in Immunology. 2014;5:1–9.
6. Gathmann B, Mahlaoui N, et al. for the ESID Registry Working Party. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. Journal of Allergy and Clinical Immunology. 2014; 134(1):116–126.
7. Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85.
8. Bonilla FA, Geha RS. Update on primary immunodeficiency diseases. Journal of Allergy and Clinical Immunology. 2006;117(2):435–41.
9. McHeyzer-Williams M, Okitsu S, Wang N, et al. Molecular programming of B cell memory. Nature Reviews Immunology. Nature Publishing Group; 2011;12(1):24.
10. Gatto D, Brink R. The germinal center reaction. Journal of Allergy and Clinical Immunology. 2010;126(5):898–907.
11. Nutt SL, and Tarlinton DM. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nature Immunology. 2011;12(6): 472–477.
12. Morita R, Schmitt N, Bentebibel SE, et al. Human Blood CXCR5+CD4+ T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets that Differentially Support Antibody Secretion. Immunity. Elsevier Inc.; 2011;34(1):108–21.
13. Crotty S. Follicular helper CD4 T cells (TFH). Annual Review of Immunology. 2011;29(1):621–63.
14. Ma CS, Wong N, Rao G, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. Journal of Allergy and Clinical Immunology. 2015;136(4):993–1006.

