Precision Medicine is changing the face of pathology
What is precision medicine?
Precision medicine (sometimes known as personalised or stratified medicine) is an emerging approach to the treatment and diagnosis of disease that takes into account variations in a patient’s genes, environment and lifestyle. It allows clinicians to better target treatments to an individual’s circumstances and aims to improve outcomes for patients. It is of particular relevance to patients with cancer and was discussed recently at the “European Association for Cancer Research/Organisation of European Cancer Institutes Joint Course: Molecular Pathology Approach to cancer”.
Molecular pathology is one of the linchpins of precision medicine with respect to the treatment of cancer patients; therapeutic decisions can no longer be made solely on the basis of histopathological diagnosis, as genomic analysis of human cancers has resulted in the identification of genetic determinants of tumour development, cancer progression and therapy response.
The use of precision medicine in cancer diagnosis and treatment
Massively-parallel sequencing or next-generation sequencing (NGS) has enabled the genetic characterisation of many cancers and identified driver genetic alterations which can be used for targeted therapies and predict treatment response.
Lung and colorectal cancer (CRC) are two of the most common cancer types, which are often diagnosed at late stages leading to limited therapeutic options. Major advances in molecular characterisation of these tumours has identified specific gene alterations such as ALK and ROS1 rearrangements and EGFR, NRAS, KRAS and BRAF mutations which are targets for specific treatment, thus increasing therapeutic options for many patients and enabling a more personalised approach to patient management.
Viapath’s Cancer Genetics service has implemented a targeted NGS panel using the Swift Biosciences Accel-Amplicon EGFR Pathway panel as part of the routine diagnostic pathway of lung and colorectal cancer, which is UKAS accredited to ISO15189 standard. The assay allows simultaneous identification of the majority of the therapeutically and prognostically relevant EGFR, KRAS, NRAS and BRAF gene variants, and is highly reproducible, giving high success rates (>95%) even for small biopsies yielding low DNA mass and significantly improving the management of patients and expanding their treatment options. For example, lung cancer patients with sensitising EGFR variants (~19%) receive treatment with EGFR tyrosine-kinase inhibitors (TKIs), and CRC patients who show no evidence of KRAS or NRAS mutations are treated with anti-EGFR monoclonal antibody therapy.
The panoply of genes with variants of known therapeutic relevance is rapidly and constantly expanding, and as identification of such variants becomes clinically relevant more comprehensive gene panels are needed in the diagnostic pathway. To meet this need the department is validating a 57 gene NGS panel for translation into routine diagnostic use. This will not only allow for detection of a larger spectrum of variants in lung and colorectal cancers but will also provide a basis for molecular diagnosis/treatment of other solid tumours.
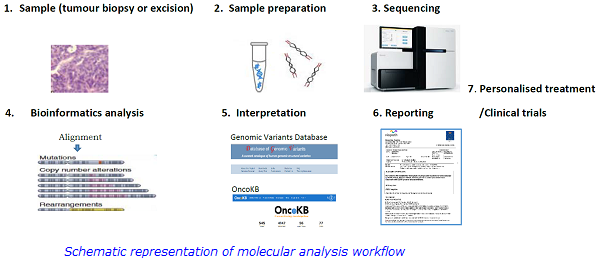
In summary, introduction of NGS and small inhibitory molecules is revolutionising molecular diagnostics and treatment strategies in cancer and has contributed to the ongoing development of personalised medicine to improve outcomes for patients with solid tumours.
For further information, please contact:
Michael Neat, Consultant Clinical Scientist:
Michael [dot] Neat [at] viapath [dot] co [dot] uk
Irina Stasevich, Clinical Scientist/Deputy Operations Lead:
Irina [dot] Stasevich [at] viapath [dot] co [dot] uk
References:
1. Hirsch FR et al. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016 Sep 3;388(10048):1012-24.
2. Mondaca S, Yaeger R. Colorectal cancer genomics and designing rational trials. Ann Transl Med. 2018 May;6(9):159.
3. Wan JCM et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017 Apr;17(4):223-238.

