Improved screening services for Candida auris
A novel screening method for Candida auris?
In Issue 4 of “pathology@viapath”, an article entitled “Candida auris: A newly discovered multi-resistant pathogen” was published. It explained that Candida auris (C. auris) is an emerging multi-drug resistant fungal pathogen, dating back to 1996 in South Korea and that there is a high mortality rate of 50-60%, associated with the fungal pathogen.
C. auris has a very high outbreak potential, especially in high risk populations, and appears to be difficult to eradicate from the environment. Hence, strict levels of infection control precautions are required, including isolation of infected patients. Screening of contacts is advised to detect early colonisation and help prevent the spread of infection.
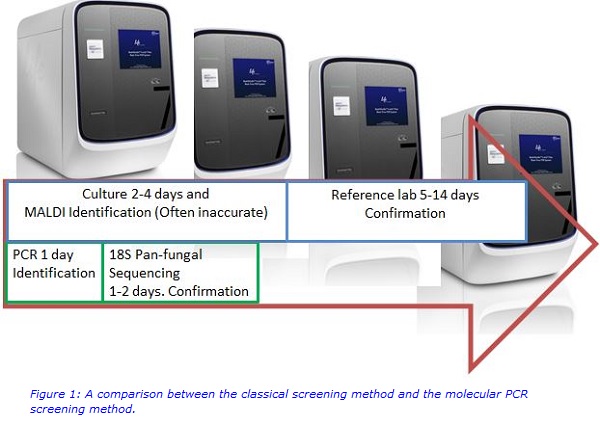
Inaccurate identification of C. auris using classical screening methods (such as Chromagar and Sabouraud’s Agar plate culture & Matrix-assisted laser desorption ionisation (MALDI) - Vitek mass spectrometry (VITEK® MS) with positives sent to the reference laboratory for MALDI-Bruker confirmation) can lead to a delay in recognising the infection or an outbreak. This can severely impact the patient outcome and also has cost implications. Thus, novel, specific diagnostic methods have been designed to overcome these limitations and improve patient care.
A new, rapid and precise C. auris polymerase chain reaction (PCR) was developed and validated to address the diagnostic screening issue. This improved the prevention measures at King’s College Hospital by allowing patients suspected of having C. auris to be de-escalated when C. auris PCR was negative and by being informative for the infection control team to know when to employ increased vigilance if patients are C. auris PCR positive, through isolation and further screening. The C. auris PCR was validated on 320 specimens using both swab sets (groin, axilla, nose, throat) and urine samples. The C. auris PCR was estimated to detect as little as [100 organisms in 1 ml] = 1 organism in 10 µl of sample (less than 0.5 Mc Farland suspension), compared to a 2 Mc Farland suspension required for the Biomerieux, Vitek®2. The preliminary sensitivity and specificity data for the novel C. auris specific PCR was determined to be greater than 95% compared with classical methods. With high positive and negative predictive values, the C. auris PCR is suitable for the current diagnostic demand.
A second, different PCR method is used as a reference PCR to check the integrity of the specimen collected. It also has an 18S pan-fungal component, which can be used to confirm the first positive C. auris detected in a patient’s specimen. The innovation of C. auris rapid detection PCR has enabled diagnostic screening methods that provide a more effective service, with a short turnaround time (24 - 48 hours) for identification (See figure 1 below).
Improving Infection Control
Viapath is offering a C. auris service that can achieve high-throughput and deliver a clinically meaningful result, in a timely manner. This service will help to better manage patients suspected of being infected with C. auris using more appropriate treatments, reduce the burden on the availability of infection control isolation rooms and help to reduce the spread of infection. Thus greatly improving infection control.
Tests run Monday to Friday. With samples received in the laboratory before 12 pm, processing is performed on the following day.
For further information on the scientific or clinical aspects of Candida auris please contact:
Dr Sharleen Braham: SBraham [at] nhs [dot] net
Dr Surabhi Taori:Surabhi [dot] Taori [at] nhs [dot] net
Or for information on the testing procedures for Candida auris, please contact:
Fearghal Tucker: Fearghal [dot] Tucker [at] nhs [dot] net
References:
- Satoh, K et al. 2009 Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 53(1):41-4.
- Lee WG et al. 2011 First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol. 49(9):3139-42.
- Kathuria, S et al 2015 Multidrug-Resistant Candida auris Misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA Sequencing and Its Antifungal Susceptibility Profile Variability by Vitek 2, CLSI Broth Microdilution, and Etest Method. J Clin Microbiol. 53(6):1823-30.
- Harrison D, et al. 2013 Development and validation of a risk model for identification of non-neutropenic, critically ill adult patients at high risk of invasive Candida infection: the Fungal Infection Risk Evaluation (FIRE) Study Feb;17(3):1-156 (http://www.viapath.co.uk/articles-and-papers/development-and-validation-of-a-risk-model-for-identification-of-non-neutropenic)

