Hepatitis E Virus: An Emerging Zoonosis
Hepatitis E: The Disease and the Virus
The hepatitis E virus (HEV) is a small virus, with a positive-sense, single-stranded ribonucleic acid (RNA) genome, that infects both humans and animals. Most individuals with HEV are asymptomatic and the infection clears completely. However, in immunocompromised patients the HEV infection may persist, potentially leading to chronic hepatitis and cirrhosis.
HEV can cause sporadic hepatitis (an inflammation of the liver) as well as large outbreaks of hepatitis and is an emerging zoonosis, for instance, crossing the species barrier from pigs to humans. Hepatitis E is widespread in Southeast Asia, northern and central Africa, India and Central America, with an increasing number of acute HEV infections being seen in Europe.
The virus is mainly spread by the faecal-oral route and the most common route of infection in the UK is from eating raw or undercooked meat, especially pork and shellfish. However, HEV can also be transmitted by blood products and a study carried out recently showed that approximately 1 in 3000 blood donors in the south of England had an HEV viraemia at the time of donation. This is likely to be an underestimate. On this basis, the Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO) has recommended that certain groups of immunocompromised patients, such as those undergoing solid organ transplantation and haematopoietic stem cell transplantation, should receive HEV negative blood components as well as monitoring for HEV infection.

Figure 1: The Rotor-gene thermal cycler used for running the assay with the different types of rotors and reaction tubes that can be used. The four different rotors give flexibility in the number of samples that can be run, ranging from 32 to 100 depending on demand.
New Test for the Hepatitis E Virus
Viapath has developed a quantitative HEV RNA real time reverse transcription polymerase chain reaction (RT-PCR) assay specifically to monitor the presence of the virus in susceptible groups of immunocompromised patients and this test will be offered as a new diagnostic service in October 2016.
In addition, Viapath will also provide HEV IgM and IgG antibody detection to help make the diagnosis of either an acute or past HEV infection in patients who may be jaundiced or have deranged liver function tests.
For further information on the scientific or clinical aspects of Hepatitis E, please contact:
Dr Mel Smith: melvyn [dot] smith [at] nhs [dot] net
Dr Mark Zuckerman: mark [dot] zuckerman [at] nhs [dot] net
Or for information on the testing procedures for Hepatitis, please contact:
Fearghal Tucker: fearghal [dot] tucker [at] nhs [dot] net
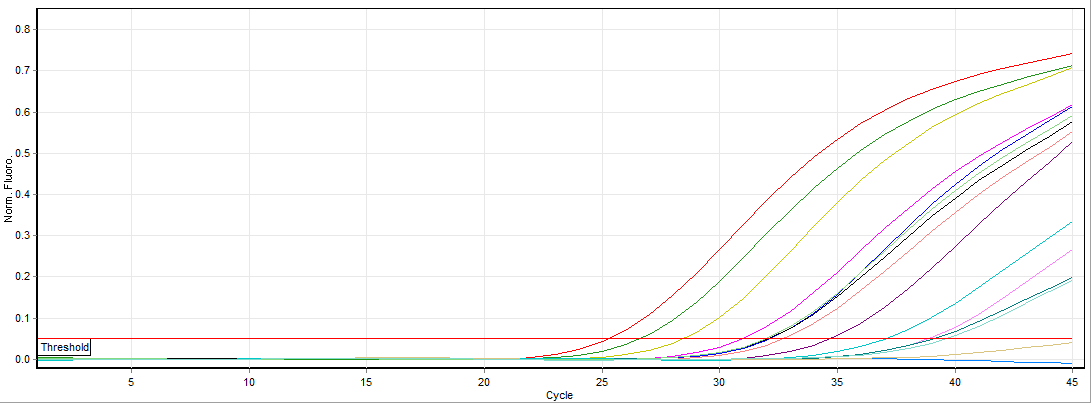 Figure 2: A typical HEV reverse-transcription PCR assay run on the Rotor-Gene showing amplification of the five quantification standards and eight samples. The horizontal red line (Threshold) set at 0.05 represents the point at which exponential amplification begins. This is the cycle at which quantification values for each sample are calculated, based on the cycle threshold values (Cts). The lower the cycle number the more viral RNA was in the extract before the RT-PCR began.
Figure 2: A typical HEV reverse-transcription PCR assay run on the Rotor-Gene showing amplification of the five quantification standards and eight samples. The horizontal red line (Threshold) set at 0.05 represents the point at which exponential amplification begins. This is the cycle at which quantification values for each sample are calculated, based on the cycle threshold values (Cts). The lower the cycle number the more viral RNA was in the extract before the RT-PCR began.

