Dihydropyrimidine dehydrogenase and fluoropyrimidine toxicity: testing times ahead
Pharmacogenetics of purine and pyrimidine drug analogues
The classic example of pharmacogenetics in clinical practice is the association between thiopurine methyltransferase deficiency and life-threatening toxicity to the immunosuppressant thiopurine drugs azathioprine and mercaptopurine. Less well known is the association between dihydropyrimidine dehydrogenase (DPD) deficiency and severe, sometimes fatal, toxicity to the fluoropyrimidine drugs 5-fluoruracil (5FU) and capecitabine, both widely used in the treatment of solid tumours including colorectal and metastatic breast cancers. The first report of severe toxicity due to DPD deficiency in a patient treated with 5FU was reported by Tuchman et al in 1985, thirty years after 5FU was developed. Whilst there is now a considerable body of peer reviewed scientific literature supporting the association between DPD deficiency and fluoropyrimidine toxicity (Lunenburg et al 2016), it has taken a further thirty years for this information to reach the popular press under the headline “How hundreds are being killed by chemo meant to save them” (Daily Mail Online. 6th October 2018).
Testing for DPD and its use in determining dosage
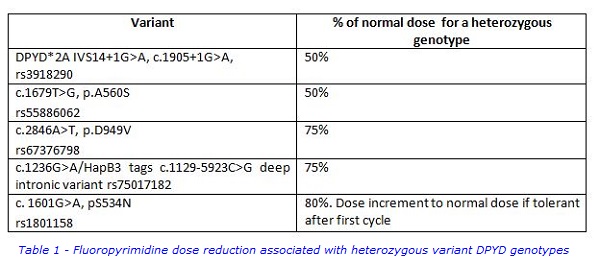
Approximately 80 to 90% of 5FU is degraded through the normal pyrimidine degradation pathway with the first step catalysed by the enzyme DPD. As 5FU has a narrow therapeutic window, impaired DPD activity leads to overdosing and toxicity. We have previously reported that four DPYD genetic variants with significant frequencies in the UK populations predict severe (grade 3 to 4) fluoropyrimidine toxicity (Loganayagam et al 2013). These four variants formed the basis of the 5FU pharmacogenetics testing panel we introduced. We subsequently included the DPYD variant c.1236G>A/HapB3 which tags the deep intronic variant c.1129-5923C>G (Meulendijks et al 2015). This variant creates a splice site leading to miss-splicing of an intronic sequence into the DPYD mRNA. All five variants in our current panel have a high penetrance, meaning that the majority of patients carrying these variants in a heterozygous genotype will experience toxicity. These DPYD variants do not however all have the same effect on DPD enzyme activity. Two variants, the splice junction variant c.1905+1G>A and the exonic variant c.1679T>G (p.A560S) result in a non-functional DPD enzyme. In a heterozygous genotype, DPD activity is 50% of normal and consequently, the fluoropyrimidine dose should also be reduced to 50% of the normal dose (Table 1). By contrast, the other three variants shown in Table 1 are associated with significant residual activity. In heterozygous genotypes, DPD activity is estimated to be 75-80% of normal and the dose given should be a corresponding 75-80% of the normal dose. Although heterozygous genotypes are most commonly seen, compound heterozygous or homozygous genotypes are found and these patients experience extreme toxicity at normal fluoropyrimidine doses. The effect of these alleles on dose reduction is additive, for example a patient homozygous for the c.1905+1G>A variant will have completely deficient DPD activity and should not be given fluoropyrimidines. The compound heterozygous genotype c.2846T/ c.1236A is associated with 50% DPD activity and would require a 50% dose reduction.
In a large Dutch study (Deenen et al 2016), testing for the variant DPYD*2A (c.1905+1G>A) only has been shown to have a marginal cost benefit. We have reported that patients experiencing severe toxicity who also carry a DPYD variant account for a disproportionate number of hospital bed days compared to DPYD wildtype patients experiencing toxicity (Loganayagam et al 2013). There is also anecdotal evidence that DPD–related toxicity is associated with prolonged hospital stays (De Sousa et al, 2015). More importantly, the Deenen study estimated death due to fluoropyrimidine toxicity in patients heterozygous for the c.1905+1G>A variant treated with a normal fluoropyrimidine dose to be approximately 10%. For some colorectal cancer patients the Daily Mail headline could equally have read “Cured by surgery, killed by chemo”.
Changing Clinical Practice
In conclusion, there is a considerable body of evidence supporting testing for DPYD variants prior to the start of fluoropyrimidine therapy to avoid severe, sometimes fatal toxicity. Patients carrying a DPYD variant should be treated with reduced dose therapy or an alternate therapy. Although there is increasing awareness of testing among patients, clinical practice has been slow to change.
For further information, please contact:
The Purine Research Laboratory, Biochemical Sciences, Viapath, St Thomas’ Hospital
Tony Marinaki (PhD):
Tony [dot] Marinaki [at] viapath [dot] co [dot] uk
Monica Arenas Hernandez (PhD):
Monica [dot] Arenas-Hernandez [at] viapath [dot] co [dot] uk
References
- Deenen MJ, Meulendijks D, Cats A, Sechterberger MK, Severens JL, Boot H, Smits PH, Rosing H, Mandigers CM, Soesan M, Beijnen JH, Schellens JH. Upfront Genotyping of DPYD*2A to Individualize Fluoropyrimidine Therapy: A Safety and Cost Analysis. J Clin Oncol. 2016 Jan 20;34(3):227-34.
- De Souza K., Papadatos-Pastos D., Karapanagiotou L., Sandri I., Marinaki A., Mansi J. DPYD genotyping as a potential cost-effective predictive biomarker of capecitabine toxicity in breast cancer. Clinical Oncology. 2015; 27(6): e12 (Abstract).
- Loganayagam A, Arenas Hernandez M, Corrigan A, Fairbanks L, Lewis CM, Harper P, Maisey N, Ross P, Sanderson JD, Marinaki AM. Pharmacogenetic variants in the DPYD, TYMS, CDA and MTHFR genes are clinically significant predictors of fluoropyrimidine toxicity. Br J Cancer. 2013 Jun 25;108(12):2505-15.
- Lunenburg CATC, Henricks LM, Guchelaar HJ, Swen JJ, Deenen MJ, Schellens JHM, Gelderblom H. Prospective DPYD genotyping to reduce the risk of fluoropyrimidine-induced severe toxicity: Ready for prime time. Eur J Cancer. 2016 Feb;54:40-48.
- Meulendijks D, Henricks LM, Sonke GS, Deenen MJ, Froehlich TK, Amstutz U, Largiadèr CR, Jennings BA, Marinaki AM, Sanderson JD, Kleibl Z, Kleiblova P, Schwab M, Zanger UM, Palles C, Tomlinson I, Gross E, van Kuilenburg AB, Punt CJ, Koopman M, Beijnen JH, Cats A, Schellens JH. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015 Dec;16(16):1639-50.
- Tuchman M, Stoeckeler JS, Kiang DT, O’Dea RF, Ramnaraine ML, Mirkin BL. Familial pyrimidinemia and pyrimidinuria associated with severe fluorouracil toxicity. N Engl J Med. 1985 Jul 25;313(4):245-9

