Clinical utility of PIVKA-II in the diagnosis of hepatocellular carcinoma
Hepatocellular carcinoma
Primary liver cancer is the third most common cause of death from cancer and the majority of cases are associated with hepatocellular carcinoma (HCC). When diagnosed with HCC, two thirds of the patients have already progressed to the advanced stage of the disease, resulting in a low survival rate. This means that the need for cost-effective and reliable early diagnostic tools is of great interest particularly as an early diagnosis followed by preventative treatment, such as liver re-section or transplantation, can markedly improve prognosis for these patients.
The use of ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) scans are essential in both diagnosis and assessment of the disease. However, the application of HCC-specific tumour markers, such as alpha-fetoprotein (AFP) and protein induced by vitamin K absence/antagonist-II (PIVKA-II), can be used as supplementary analysis to aid the diagnosis and monitoring of confirmed HCC cases. AFP is currently the most common marker used; however, it has been shown to lack both sensitivity and specificity. Hence, the novel tumour marker PIVKA-II is becoming a more attractive alternative with its proven high sensitivity and greater specificity for HCC.
The nature of PIVKA-II
PIVKA-II is an abnormal form of prothrombin, a precursor protein made in the liver in the presence of vitamin K, that plays a central role in the blood-clotting process. Under normal conditions, prothrombin undergoes post-translational modification, a process where particular enzymes add functional groups to a protein to determine its final structure and, hence, its function. Prothrombin is subjected to a post-translational γ-carboxylation reaction by the γ-glutamyl carboxylase enzyme. However, in the absence of vitamin K, the enzyme is unable to function. Thus, PIVKA-II is formed which ultimately leads to the loss of the biological activity of prothrombin. Interestingly, in some cases carboxylation may not occur at all, resulting in the formation of PIVKA-II variants that each display different degrees of biological activity.
The role of PIVKA-II in the pathology of HCC
Although the relationship between PIVKA-II and the occurrence of HCC is still not well defined, it has been shown that PIVKA-II can promote an increased rate of cell proliferation as it has the ability to mimic the structure of hepatocyte growth factor (HGF). HGF has the primary function of regulating the growth of hepatocytes (liver cells); however, in the presence of a mimicking protein like PIVKA-II, this can result in uncontrolled hepatocyte proliferation, a signifying characteristic of HCC. Furthermore, PIVKA-II promotes angiogenesis (the formation of new blood vessels) in HCC, resulting in tumour invasion of liver tissue as well as metastases (secondary malignant tumour growths). HCC metastasis occurs via the stimulation of vascular endothelial growth factor (VEGF) which allows the formation of vasculature to supply the growing tumour with oxygenated blood, and epidermal growth factor (EGF) which enables tumour cells to grow, proliferate and differentiate.
Use of the PIVKA-II test to determine presence of HCC
Method
In this study, 87 samples were analysed for PIVKA-II from three groups of patients:
· Group A – randomly-selected patients with non-HCC pathology of the liver such as viral cirrhosis and hepatitis
· Group B – patients with changes to the liver suggestive of possible HCC discovered in the course of US/MRI/CT investigations
· Group C – patients with diagnosed HCC at different stages, where diagnosis was established in the course of histological examination of liver biopsy samples
The analysis was performed by chemiluminescent microparticle immunoassay (CMIA) using a two-step sandwich reaction where a binding reaction occurs between antibodies against PIVKA-II and specific binding sites (epitopes) on PIVKA-II in the patient sample, followed by a washing stage, with the subsequent addition of chemiluminescent labels, resulting in the emission of light. This enables the quantification of PIVKA-II concentration in the tested sample.
Results
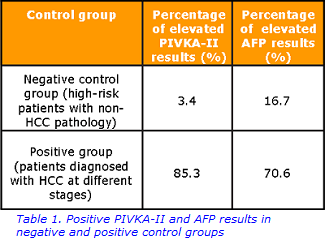
In the positive group, PIVKA-II was elevated in 85.3% of the patients; normal PIVKA-II levels detected in this group can be explained by the normalisation of PIVKA-II concentration after treatment (Table 1). In contrast, an elevated PIVKA-II concentration was found in just one patient from the negative control group; this result may be interpreted as either false positive (elevation of PIVKA-II due to non-HCC pathology) or true positive (the patient would need to undergo more comprehensive screening to confirm the presence of HCC).
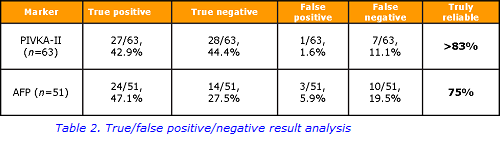
In this study, the analysis revealed that more than 83% of PIVKA-II results were reliable, in comparison with 74.6% of AFP results showing true diagnostic value (Table 2). Taking into account considerable difference between sensitivity and specificity rates for PIVKA-II and AFP (79.4 vs 96.6% and 70.6 vs 82.4% respectively), allows the conclusion that PIVKA-II displays slightly better clinical utility in HCC diagnosis.
Conclusion
PIVKA-II has several advantages over AFP in terms of clinical utility for HCC diagnosis and prognosis: it is more sensitive to small HCC tumours, correlates with HCC progression significantly better and has a shorter half-life than AFP, making it more suitable for monitoring the cancer. However, there are some limitations; one of these being its vulnerability to the action of potentially interfering pharmacological agents such as warfarin and certain antibiotics. Therefore, the combination of PIVKA-II and AFP is suggested to be the best option for highly accurate laboratory diagnosis of HCC, supplementary to imaging techniques.
For further information, please contact:
Human Nutristasis Laboratory: 0207188 6815 / 89543
Acknowledgement:
This article has been adapted, by Parmilla Dhamrait, from a paper written by Volha Klimovich, Kieran Voong, Roy Sherwood and Dominic Harrington that appeared in Clinical Laboratory, June 2017
References
McMasters K, Vauthey J. Hepatocellular carcinoma: targeted therapy and multidisciplinary care. Springer 2011; Chapters 1–5, 8.
Ji J, et al. Diagnostic evaluation of des-gammacarboxy prothrombin versus α-fetoprotein for hepatitis B virus-related hepatocellular carcinoma in China: a large-scale, multicentre study. PLoS One 2016; 11: e0153227.
Lim TS, et al. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol 2015; 51: 344–353.
De J, et al. A systematic review of des-γ-carboxy prothrombin for the diagnosis of primary hepatocellular carcinoma. Medicine 2016; 95: e3448.
Kokudo M, et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 2015; 45: 123–127

