Cervical Cancer Screening in England – The journey so far
Cervical cancer prevention has been based on cervical cytology screening for the last 50 years and has been a major success in the UK. With recent rapid advances in understanding of human papillomavirus (HPV), its role in carcinogenesis, the clinical applications of primary prevention by HPV immunisation and secondary prevention by HR-HPV testing, the approach to cervical cancer prevention in the UK is undergoing significant change.
In the beginning…….
Screening for cervical cancer has played a significant role in medicine since the late 19th century, when public health authorities identified the need to detect specific diseases in certain groups of the population. However, it is only relatively recently that screening for the early detection of cancer has gained widespread acceptance. Fundamental to this was the pioneering work in the development of techniques of exfoliative cytology initiated by Dr George Papanicolaou who, in 1941, described how this test could be of value in the early detection of cervical cancer. He went onto describe techniques to sample, process and stain cells to identify precancerous changes and became known as “The Father of Cytology”.

The “Pap” test (or “Pap” smear), named after Dr Papanicolaou, had all the criteria of a screening technique for cancer, as described by Cochrane and Holland in 1971 who recommended:
- Simplicity – The sample should be easy to take.
- Acceptability – The method of sampling must be acceptable to the population involved.
- Accuracy – The test results must give a true measurement of the disease under investigation.
- Cost – The expense of screening must be considered in relation to benefits of early detection of disease.
- Precision – The tests must give consistent results in repeated trials.
- Sensitivity – The test should be able to detect all individuals with the disease.
- Specificity – The test should not identify positive results in non-diseased individuals.
Early screening in England
In 1964, cervical screening was introduced in England but it was quite disorganised. Cervical “smears” were taken from many women, most opportunistically, but abnormal results were infrequently followed-up. Policies regarding which and how women should be screened were inadequate. Harsh treatment regimes coupled with no classification system for cellular changes increased the risk of over-diagnosis and overtreatment.
Mortality rates from cervical cancer in women <35 years old rose alarmingly leading to increased awareness of the limitations of cervical screening. By the mid 1980’s a rise in cervical abnormalities resulted in backlogs which created further public dissatisfaction with the cervical screening service. Reports emerged of organized, public-health centred screening programmes using a population-based registry to recall women which had successfully reduced mortality.
The birth of the NHS cervical screening programme (NHSCSP)
In 1988 a centralised NHS cervical screening programme was launched. Pivotal to this was a centrally agreed policy regarding who to screen and treat. The Department of Health (DOH) requested district health authorities to implement a computerised call/recall system. Women aged 20-64 years were invited to participate in cervical cancer screening every 3-5 years, leading to a significant increase in the number of women tested.
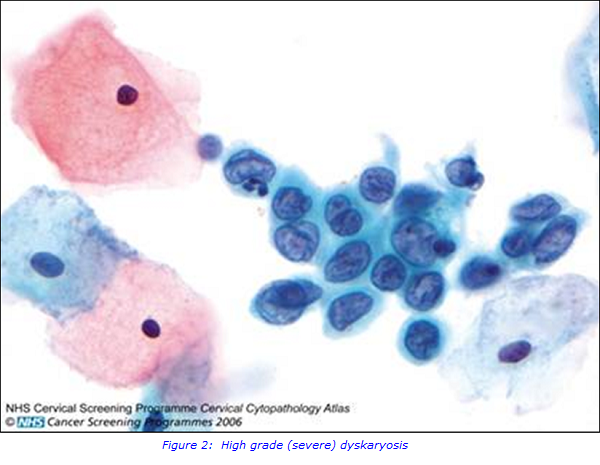
However, inadequate samples comprised 7-9% of total tests, requiring tests to be repeated and 3 consecutive inadequate test results required referral to colposcopy (a simple procedure used to look at the cervix), due to the increased risk of undetected cancerous precursors. 5-10% of tests were categorised as borderline changes of mild precancerous changes (dyskaryosis), 1% had moderate dyskaryosis, and 0.5% had severe dyskaryosis. Treatment options for moderate and severe dyskaryosis were clear. However, treatment options for borderline changes and mild dyskaryosis ranged from cytological surveillance to referral for colposcopy.
A series of errors within the cervical screening programme identified the need for external quality assurance and proficiency testing. One widely publicised failure occurred at Kent and Canterbury Hospitals NHS Trust; inadequate training of screening staff and poor supervision had been raised. These concerns were acknowledged, which led to 90,000 tests being re-examined. A public enquiry followed which recommended the reinforcement of quality assurance protocols within the NHSCSP. Responsibility for screening was placed with the directors of public health, which prompted the government to implement a mandatory accreditation system for all cervical screening laboratories in the UK.
Advances in technology drive change
During 2000-2010, the advent of Liquid-Based Cytology (LBC) (which involves making a suspension of cells from a sample and producing a thin layer of cells on a slide) presented opportunities to evaluate the role of high risk HPV (HR-HPV) testing as well as the potential to automate the screening process. Tests introduced into the NHSCSP are subject to rigorous evaluation and this suggested that LBC may reduce inadequate rates, improve sensitivity and reduce the time taken to screen a sample. Three pilot sites were set up and run from 2001-2002 in England, with the conclusion that LBC had comparable sensitivity and specificity to conventional cytology, but led to far fewer inadequate results, requiring fewer repeat tests and was cost-effective. National rollout was implemented in 2003 after endorsement by NICE (National Institute of Clinical Excellence) and completed in 2008.
LBC provided a means for additional reflex testing for HR-HPV which facilitated triage of women with low grade abnormalities who were at greater risk of developing cervical cancer and hence required referral to colposcopy. Triage of low grade abnormalities via HR-HPV testing reduced the number of repeat tests, however increased the number of referrals to colposcopy. A refined protocol was used for a national rollout, which included an HR-HPV as a test of cure (TOC) for post treatment follow-up to identify HR-HPV negative women who can be safety returned to routine recall, instead of annual cytology follow-up for 10 years. This utilises the high negative predictive value of HR-HPV testing to identify women of rare risk of CIN2 or worse. In 2011, Advisory Committee for cervical screening recommended to the government that HR-HPV test for triage and TOC be implemented as this leads to a “more patient-centred service and major cost saving”. Rollout concluded in 2014.
In 2003, based on evidence suggesting that cervical screening was not as effective in women under 25 years old, a key policy change was to raise the age at which screening commences to 25. This was reviewed by the Advisory Committee in 2009, due to increased public pressure to reduce the age to 20 following the publicity surrounding the death of Jade Goody from cervical cancer. However, evidence showing that screening had little or no benefit to women under 25 years old, resulted in the age not being lowered.
In 2003, the screening frequency for routine recall was increased for women aged 50-64 to 5 yearly, based on an audit which concluded that 5 yearly screening provided considerable protection for this age group.
In 2007, the Cancer Reform Strategy announced that all women should receive their cervical screening tests results within 2 weeks of sampling and that this would be implemented by 2010. This metric was added as a vital sign of the operating framework for the NHS in England 2010/11. Laboratory re-configuration was recommended to produce larger more efficient laboratories alongside larger call/recall centres which was backed by Lord Carter of Coles’ independent review of NHS pathology services in 2016.

Where do we go now?
HR-HPV primary screening had been tested in the HPV pilot sites since HPV triage and TOC rollout. In 2016, the UK National screening committee recommended that HPV primary screening should be adopted by the NHSCSP and an announcement was made that HPV primary testing would be implemented in England by 2019.
Primary HR-HPV screening is a complex pathway which will use the HR-HPV test as the primary test. Only tests with a positive HR-HPV result will have a slide produced for reflex cervical screening, reducing the current volume of cervical cytology work by approximately 87%.
The HPV primary screening implementation group recommended, in 2017, that there should be 5-15 cytology screening centres linked to HPV test centres throughout the UK. These future centres will require robust IT systems to support them. Staff training and future workforce requirements are being carefully considered in light of the impact of primary screening on the workforce, with staff retention being a significant concern. Now NHS England, who commission the NHSCSP, is working through how this preferred service model can be implemented by 2019.
For further information please contact:
Suzanne Ferrá
Cervical cytology Operations Manager
020 7188 2905
Suzanne [dot] ferra [at] viapath [dot] co [dot] uk
References:
M. Bibbo. Comprehensive Cytology. W.B. Saunders Company: 2nd edition, 1997.
R. Albrow, H. Kitchener, N. Gupta, M. Desai. Cervical screening in England: The past, present and future. Cancer Cytopathology.120: 87-96 2012.
R. Stubbs. HPV primary screening in the cervical screening programme. Public Health England: April 2016.
R. Stubbs. Deciding how best to roll out HPV testing as the primary cervical screening test. Public Health England: January 2017.
T. Freeman-Wang, M.E. Cruickshank, H.C.Kitchener. Progress in Cervical Screening in the UK. Royal College of Obstetricians and Gynaecologists. Scientific impact paper 7, March 2016.
Cancer Research UK. HPV and Cancer. www.cancerresearchuk.org/about-cancer/causes-of-cancer/infections-hpv-an..., March 2017.

